Decontamination of reusable non-invasive Care Equipment
Patient care equipment is easily contaminated with blood, body fluids, secretions, excretions and infectious agents, and if not decontaminated, they can be easily transferred microorganisms during care delivery. Therefore, all reusable, non-invasive items must be decontaminated to prevent cross-infection. Decontamination includes the entire process, i.e., cleaning, disinfection, and/or sterilisation.
All healthcare facilities (HCFs) must have a comprehensive decontamination policy to ensure that all reusable medical items and equipment are adequately cleaned, disinfected, and/or sterilised before re-use. The policy should be based on the manufacturer’s instruction and be drawn up in consultation with the IPC team, Sterile Service Department, and Decontamination Lead.
Staff responsible for the Decontamination of care equipment must have adequate practical training. The training must be updated regularly and when new equipment is purchased. The head of the department must keep records of training. All personnel responsible for cleaning, handling, and reprocessing contaminated items must receive appropriate vaccinations.
It is also the responsibility of each healthcare worker not to allow unsafe equipment or devices on any patient. Therefore, all staff should be trained to check the safety of any medical device before using it.
Before using any sterile equipment, the staff must check the expiry date and ensure that the packaging is intact and there are no apparent signs of packaging contamination. The items have sterility indicators consistent with the successful completion process.
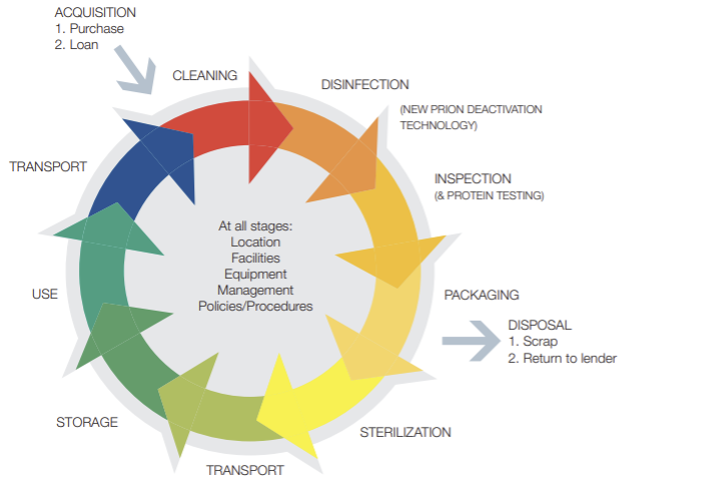
Figure 1 The decontamination cycle of reusable surgical instrument
Source: HTM01-01 decontamination of surgical instruments
Risk Assessment of Contaminated Items
The risk of transferring microorganisms from instruments and equipment is dependent on the following factors:
- The presence of microorganisms, their number, and their virulence
- The type of procedure that is going to be performed (invasive or non-invasive)
- The body site where the instrument or equipment will be used
In 1968, Earle Spaulding devised a classification of medical devices and equipment based on the degree of risk of infection from these items.
- High (Critical): Medical devices involve breaking the skin or mucus membrane or entering a sterile body cavity or bloodstream, e.g. surgical instruments, implants, prostheses and devices, urinary catheters, cardiac catheters, needles and syringes, dressings, sutures, delivery sets, dental instruments, rigid bronchoscopes, cystoscopes etc. These items must be sterilised.
- Intermediate (semi-critical): Medical devices in contact with mucous membranes or non-intact skin,g. reusable bedpans and urinals, breast pumps, etc. After thoroughly cleaning, these items require high-level disinfection by heat or chemicals.
- Low (non-critical): These items are in contact with intact skin, e.g. stethoscopes, ECG lead, non-invasive ultrasound probes, etc. These items require low-level disinfection as per the local decontamination policy.
Classification of Equipment
- Single-use: This equipment is used once on a patient and then discarded. This equipment must never be re-used. The packaging will carry this symbol (right).
 Single patients use equipment that can be re-used on the same patient and may require Decontamination in between use, such as nebuliser masks. Needles and syringes are single-use devices and must never be used more than once or re-used to draw up additional medication (see Injection safety section. Other reusable invasive equipment is used once, then decontaminated at the sterile services department, e.g. surgical instruments (See Fig.1 ).
Single patients use equipment that can be re-used on the same patient and may require Decontamination in between use, such as nebuliser masks. Needles and syringes are single-use devices and must never be used more than once or re-used to draw up additional medication (see Injection safety section. Other reusable invasive equipment is used once, then decontaminated at the sterile services department, e.g. surgical instruments (See Fig.1 ).
- Reusable non-invasive equipment: They are often called communal equipment. They are re-used on more than one patient. Decontamination of reusable, non-invasive care equipment must be undertaken between each use and after blood and/or body fluid contamination as per manufacturers’ instructions. After Decontamination, they must stored clean and dry.
- Always adhere to COSHH risk assessments and manufacturers’ guidance for using and decontaminating all care equipment.
- As part of an equipment cleaning protocol, they should be decontaminated at regular predefined intervals.
- If providing domiciliary care, equipment should be transported safely and decontaminated as above before leaving the patient’s home.
- An equipment decontamination status certificate will be required if any item of equipment is being sent to a third party, e.g. for inspection, servicing or repair. These items must be decontaminated before inspection, servicing or repair.
Flow chart for the Decontamination of reusable, non-invasive care equipment
Literature Review
- Management of Care Equipment, NHS Services, Scotland.
- https://www.nipcm.hps.scot.nhs.uk/resources/evidence-and-research/
References
- HTM 01-01 Decontamination of surgical instruments.
- Spaulding EH. Chemical disinfection of medical and surgical materials. In: Lawrence CA, Block SS, (eds). Disinfection, Sterilisation and Preservation. Philadelphia: Lea & Febiger, 1968, pp. 517–531.
