Transmission Based Precautions
Introduction
Transmission-based precautions are additional to standard precautions for patients with suspected or known transmissible infection or colonisation with multi-drug resistant organisms (MDROs). They should be applied when an infectious disease is suspected without waiting for Laboratory confirmation.
Patients with certain conditions must be isolated immediately, for example:
- Diarrhoea and/or vomiting
- Undiagnosed rashes, fevers or respiratory symptoms
- Known or suspected infection or colonisation with MDROs, e.g. Carbapenem Producing Enterobacteriaceae (CPE), MRSA
- Suspected or confirmed Group A streptococcal infection (e.g., necrotising fasciitis)
- Patients admitted from other hospitals within and outside the UK who may be infected/colonised with (MDROs) or other endemic infections.
Risk Patient and Placement
Applications of transmission-based precautions should be considered within the framework of the hierarchy of controls and risk assessment. Clinical judgement and decisions should be made by staff on what additional precautions are required and should be based on the suspected/known microorganisms, transmission mode, virulence of suspected pathogens, care setting, and procedures undertaken (Refer to Triage, Risk Assessment, and Patient Placement).
Isolation facilities
The objective of source isolation is to isolate the infected/colonised patient in a single room with an en-suite toilet facility. The old term’ barrier/precaution nursing’ should be avoided. The type of additional precautions assigned is based on the mode of transmission and is categorised into contact, droplet, or airborne (Refer to Basic Concepts).
Single room isolation in hospital settings
Single room: Source isolation can be achieved by nursing the patient in an en-suite single room with hand-washing facilities. Isolation facilities should be prioritised depending on the known/suspected infectious agent. The clinical judgement and expertise of the staff involved in a patient’s management and the advice of the infection Prevention and Control (IPC) team should be sought, particularly for isolation prioritisation, when single rooms are in short supply. All patient placement decisions, including IPC risk assessments and isolation requirements, must be documented in the patient notes and communicated during handovers with other healthcare/care providers.
Airborne infection isolation room: In a hospital setting, airborne infection isolation rooms are used for infections transmitted by the airborne route, which means air flows from the corridor to inside the patient room, and 6 to 12 air exchanges per hour are required. The exhausted air goes outside the building, away from places where people walk or congregate and any air intake openings. The door is kept closed when not required for entry and exit. If an airborne infection isolation room (AIIR) is unavailable, the patient(s) should be placed in a well-ventilated room with the door closed.
Cohort: If a single room is unavailable, cohort patients with similar symptoms and diagnosis and/or infected or colonised with the same microorganism. Cohorting should only be done on the advice of the local IPC team and/or Public Health Agency, and a dedicated staff team should care for these patients; however, this can only be implemented if sufficient staff are available. They are separated by at least 3 feet (1 metre) with the door closed, if feasible.
Door Signage: To communicate isolation requirements and prevent unnecessary visitors and non-essential staff entry, signage on doors/areas should be used. Isolation room doors should remain closed. If this is not possible, e.g., in paediatrics, there should be a documented risk assessment. Patient confidentiality must be maintained.
Transfer of Patient: Infectious patients should only be transferred to other departments if clinically necessary. If the patient’s infectious agent is transmitted through the airborne/ droplet route, the patient should wear a surgical face mask in communal areas during transfer, if tolerable. The receiving department/hospital and transporting staff must know the necessary IPC precautions.
Isolation of patients in care settings
In community care settings with or without nursing care, the infected residents should remain in their bedrooms and keep the doors closed. If unable to isolate, this should be documented. Avoid unnecessary transfer of residents within/ between care areas. If it’s necessary to transfer to the hospital, ambulance services and the hospital admission area should be informed of the resident’s infectious status. It is recommended to seek advice on the resident’s clinical management from the GP and the Public Health Agency.
Primary care and outpatient settings
Patients with suspected/known infection or colonisation with MDROs should be prioritised for assessment and treatment in primary care and outpatient settings. The scheduled appointments are to be made either at the beginning or the end of the clinic session. While awaiting assessment and during care management, infectious patients should be separated from other patients by at least 3 feet (1m). If it is necessary to transfer from a primary care facility to the hospital, ambulance services should be informed of the patient’s infectious status.
Management of patient care equipment
If possible, use single-use items in an isolation room/cohort area. Reusable non-invasive care equipment should be dedicated to the isolation room/cohort area. These items/ equipment must be decontaminated before use on another patient. When using reusable non-invasive care equipment in isolation/cohort areas, it is important to increase the frequency of decontamination. For how to decontaminate non-invasive reusable equipment (Refer to Flow chart)
Safe Management of the care environment
Patient isolation/cohort rooms/areas should be thoroughly cleaned and disinfected at least daily, and the IPC team may recommend increasing the frequency. In addition, increased frequency of cleaning and disinfection schedules should be incorporated into the environmental decontamination schedules for areas with higher environmental contamination rates (toilets, commodes, etc.), particularly if patients have diarrhoea. In addition, special attention must be paid to frequently touched surfaces (door and toilet handles, locker tops, over-bed tables, bed rails, etc.). All areas and rooms must be cleaned from highest to lowest points and from least to most contaminated points. Refer to the National Cleaning Standards for enhanced cleaning in different settings.
Terminal cleaning after discharge/death of infected patients
Terminal cleaning is performed after the infected/colonised patient’s discharge/death or the end of an outbreak. The process involves cleaning the environment thoroughly, disinfecting high-frequency hand-touched surfaces, and decontaminating all reusable items and equipment with the appropriate disinfectant. The IPC team and the nurse or manager in charge of the ward/unit/facility will discuss and give advice. The terminal cleaning should not commence until the relevant room/area is fully vacated. There is no requirement for terminal cleaning of outpatient/theatre recovery unless advised by the IPC team.
Procedure for terminal cleaning
- Gather all equipment required for the terminal clean at the point of use, e.g. mop bucket, mop, disposable colour-coded cloths, disposable roll, yellow clinical waste bags & tags, alginate & red bags, wet floor sign, etc.
- Don Personal Protective Equipment (PPE), i.e. disposable apron and gloves, before entering the room
- Discard all single-use disposable items in the room as Clinical Waste and bagged before removal from the room.
- Bedding/bed screens/curtains – manage as infectious linen and bagged before removal from the room.
- Reusable non-invasive care equipment requires decontamination in the room before removal.
- Prepare and dilute detergent and disinfectant solution as per the manufacturer’s instructions.
- Ventilation of the area/room being cleaned must be adequate; if there is no window, the door should be left open, especially when applying hypochlorite solutions.
- Use disposable cloths/paper rolls for cleaning during the terminal cleaning. Use disposable mop heads or decontaminated mop heads after cleaning as appropriate.
- After cleaning, if rinsing is required, use water for rinsing before drying. In particular, it is essential to rinse chlorine-containing solutions from stainless steel surfaces to prevent corrosion.
- Ensure that PPE is changed when moving from one room/area to another and disposed of into a clinical waste bag.
- Always decontaminate your hands after removing and disposing of PPE.
After Aerosol Generating Procedures
After aerosol-generating procedures (AGP), the vacated room must be decontaminated. The room’s ventilation and air change within the room determines the clearance of infectious particles after an AGP. In hospitals where most of these procedures occur, it takes at least 20 minutes to clear the air of infectious particles. The recommended air changes in the hospital should be at least six air changes per hour in general wards and single rooms and a minimum of 10 air changes per hour in negative-pressure isolation rooms or airborne infection isolation rooms. Seek advice from your local IPC team.
Note: Some healthcare facilities use other technologies, such as steam, vaporised hydrogen peroxide, or ultraviolet light. This is an additional step in cleaning but should not replace the physical cleaning and/or disinfectant of the environment, items and equipment.
Chemical Disinfectant: Follow local protocol. Control of Substances Hazardous to Health (COSHH) regulations must be followed when using chemical disinfectants. Manufacturers’ guidance and recommended product ‘contact time’ must be followed for detergent and disinfectant solutions. For the disinfection of items and equipment, please follow local guidelines and the manufacturer’s instructions. Do not mix chemicals; only use a cleaning product provided by your employer.
Sodium hypochlorite (bleach) solution: They have a rapid action and broad spectrum. They have good activity against bacteria, mycobacteria, spores, and enveloped and non-enveloped viruses. Since undiluted bleach solutions are available in various concentrations, test strips are available to check the concentration to measure the level of available chlorine. The recommended concentration for general environmental disinfection is 1,000 parts per million (ppm). Alternatively, using Sodium dichloro-isocyanurate (NaDCC) tablets is recommended to achieve the recommended concentration by adding tablets per the manufacturer’s instructions in water to achieve 1000 ppm. It is important to note that bleach solutions are also corrosive to metals and unsuitable for the disinfection of certain items and equipment. In addition, they are inactivated by organic materials; therefore, cleaning before disinfection is essential. They are not a stable solution, and once prepared, they should be used on the same day. They should be used in a well-ventilated area as they are respiratory irritants. Use PPE, as they can cause skin irritation and allergic reactions.
A combined detergent/disinfectant solution at a dilution of 1,000 parts per million available chlorine (ppm available chlorine (av.cl.) or a general-purpose neutral detergent in warm water followed by a solution of 1,000 ppm av cl. or alternative locally agreed cleaning product. However, for cleaning for C. difficile (spore-forming), a two-step process is required, i.e., a rigorous mechanical cleaning process (e.g., using friction) followed by disinfectants with sporicidal properties, e.g. sodium hypochlorite solution 1,000 ppm or 5,000ppm (CDC/ICAN, 2019)
Aerosol-generating procedures
Aerosol-generating procedures (AGPs) are medical procedures that can release aerosols from the respiratory tract. The criteria for an AGP are a high risk of aerosol generation and an increased risk of transmission from patients with a known or suspected respiratory infection. Refer to Box for the list of medical procedures.
Aerosol-generating procedures |
• Awake* bronchoscopy (including awake tracheal intubation) • Awake* ear, nose, and throat (ENT) airway procedures that involve respiratory suctioning • Awake* upper gastrointestinal endoscopy • Dental procedures where high-speed or high-frequency devices, e.g. ultrasonic scalers/high-speed drills are used • Induction of sputum • Respiratory tract suctioning** • Surgery or post-mortem procedures (like high-speed cutting/drilling) are likely produce Aerosol from the respiratory tract (upper or lower) or sinuses. • Tracheostomy procedures for insertion or removal. |
*Awake, including ‘conscious’ sedation (excluding anaesthetised patients with secured airway)
** The available evidence relating to respiratory tract suctioning is associated with ventilation. In line with a precautionary approach, open suctioning of the respiratory tract, regardless of association with ventilation, has been incorporated into the current (COVID-19) AGP list. The consensus of the UK IPC cell is that only open suctioning beyond the oro-pharynx is currently considered an AGP; oral/pharyngeal suctioning is not an AGP.
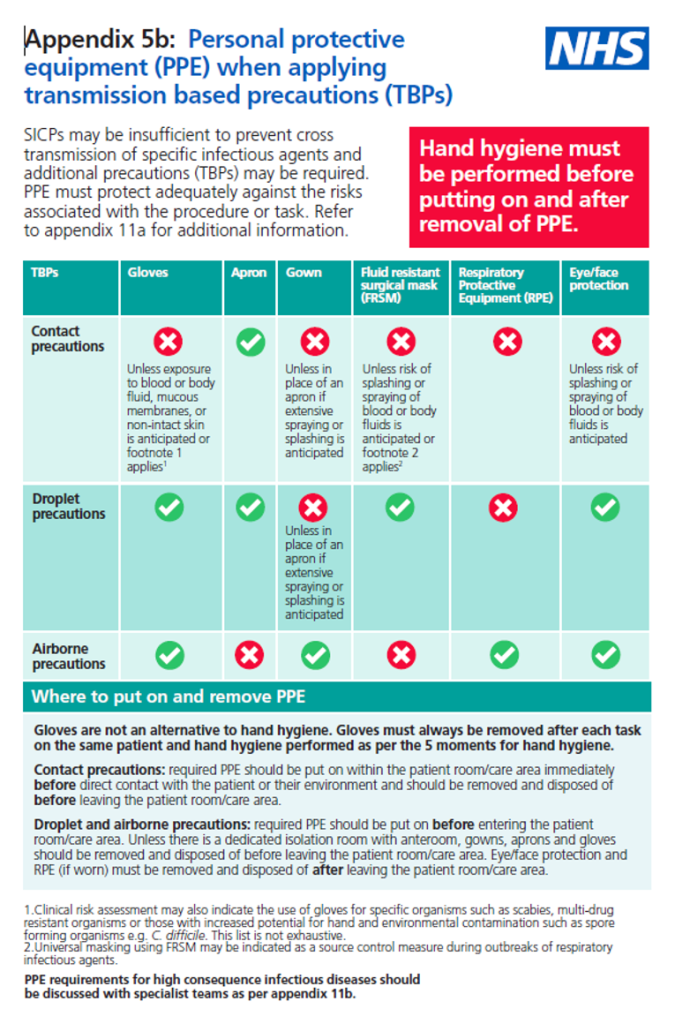
Personal protective equipment (PPE)
Personal Protective Equipment (PPE) must still be used following standard precautions (Refer to Personal Protective Equipment).
Respiratory protective equipment (RPE)/Respirators (FFP3)
Respiratory Protective Equipment (RPE), i.e., a filtering facepiece (FFP), must be considered when a patient is admitted with a known/suspected infectious agent/disease spread wholly or partly by the airborne route and when carrying out aerosol-generating procedures (AGPs; see 3.4) on patients with a known/suspected infectious agent spread wholly or partly by the airborne or droplet route. For a list of organisms spread wholly or partly by airborne (Aerosol) or droplet routes, please refer to Table in section 5.
Respiratory protection aims to protect the wearer from pathogens spread by the airborne route, e.g. measles, chickenpox, tuberculosis, and when performing aerosol-generating procedures (See 3.4) on patients with suspected or known respiratory tract infections. The decision to wear an FFP3 respirator/hood should be based on clinical risk assessment.
Healthcare workers who may require protection using an FFP3 respirator mask should be fit-tested before use and receive training on the use of the mask. Fit-testing is conducted by the Occupational Health Department. Individuals are responsible for checking the seal on their mask before each use. FFP3 respirator masks should be changed after every use or when visibly soiled. They should also be changed if breathing becomes difficult.
Staff in primary care/outpatient settings or care homes would not usually be required to wear an FFP3 respirator for routine care unless an AGP is performed when staff should wear a fit-tested FFP3 respirator.
According to the UKHSA, the following risk categorisation is the minimum requirement for staff groups that require FFP3 fit testing. Healthcare organisations can add to this, for example, where there are high-risk units. This categorisation is inclusive of out-of-hours services.
Level 1 – Preparedness for business as usual
Staff in clinical areas most likely to provide care to patients who present at healthcare facilities with an infectious pathogen spread by the airborne route and/or undertake aerosol-generating procedures, i.e., A&E, ICU, paediatrics, respiratory, infectious diseases, anaesthesia, theatres, chest physiotherapists, Special Operations Response Team (Ambulance), A&E, ambulance staff, bronchoscopy staff, resuscitation teams, mortuary staff.
Level 2 – Preparedness in the event of an emerging threat
Staff in clinical settings are likely to provide care to patients admitted to the hospital in the event of an emerging threat, e.g. Medical receiving, Surgical, Midwifery and Specialty wards, and all other ambulance transport staff. In an Epidemic/Pandemic, ‘Local Assessment as per the organisation’s preparedness plans apply.
FFP3 respirator or powered respirator hood
Powered respirator hoods are an alternative to tight-fitting FFP3 respirators. For example, when fit testing cannot be achieved, powered hoods can be single-use (disposable) or reusable (with a decontamination schedule, see note) and must be fluid-resistant; the filter.
They may be considered for use by visitors if there has been no previous exposure to the infected person or infectious agent, but must never be worn by an infectious patient(s) due to the nature of the respirator filtration of the incoming air, not expelled air. They must be enclosed with the exterior, and the belt can withstand disinfection with 10,000ppm av. cl.
Valved respirators are not suitable for use when sterility over the surgical field is required (unless they are shrouded), and this must be assessed locally, e.g., in theatres. All tight fitting RPE ie, FFP3 respirators must be:
- Single-use, disposable or reusable, and preferably fluid-resistant. If not, a full-face visor should be worn.
- Fit tested on all healthcare staff who may be required to wear a respirator to ensure an adequate seal/fit as per the manufacturer’s guidance.
- Fit checked every time a respirator is donned to ensure an adequate seal has been achieved per the manufacturer’s instructions.
- Compatible with other facial protection used, i.e. protective eyewear, so that this does not interfere with the seal of the respiratory protection. Regular corrective spectacles are not considered adequate eye protection.
For any facial hair, the hair must not cross or interfere with the respirator sealing surface. If the respirator has an exhalation valve, hair within the sealed mask area should not impinge upon or contact the valve. You must pass a face fit test for any tight-fitting respiratory protective equipment you need for work activities. Examples of facial hair styles compatible with FFP3 respirators can be found here.
Please note: reusable respirators, including powered respirator hoods, can be utilised by individuals if they comply with HSE recommendations. These should be decontaminated and maintained according to the manufacturer’s instructions.
Further information regarding fitting and fit checking of respirators can be found on the Health and Safety Executive website at: Respiratory protective equipment (RPE) (hse.gov.uk)
Removal (doffing) of PPE
In the absence of an anteroom/lobby, remove FFP3 respirators and eye/face protection in a safe area (e.g. outside the isolation/cohort room/area). All other PPE should be removed in the patient care area.
For the recommended method of putting on and removing PPE, see UKHSA guides.
Further information can be found in the Respiratory Protective Equipment (RPE) literature review and the PPE for Infectious Diseases of High Consequence (IDHC) literature review.
https://www.nipcm.hps.scot.nhs.uk/resources/evidence-and-research/
Infection prevention and control when caring for the deceased
If the person has died of an infectious disease, the risk of transmitting infection is usually less than for living patients. Although pathogens may have infected a recently dead person, most microorganisms that cause death do not survive for long and stop multiplying and die rapidly in a dead body due to microbial competition as the body decomposes. However, soft tissues remaining on a cadaver could present an infection risk, and infection occurs mainly from contact with the infected body and any fluids that leak from it. Therefore, the risk of infection is increased when procedures, e.g., embalming or post-mortem, are performed. In such cases, the transmission of infection can occur via contact with blood and body fluids, sharps injury, exposure of infectious materials via broken skin, and through splashes of blood and body fluids to the mucous membrane of the eyes, nose, and mouth. Therefore, all staff handling the body e,g mortuary staff, funeral directors, or embalmers must be informed of any infection risk.
Regardless of the infectious agent, when dealing with a recently dead body, the risk of acquiring infection can be significantly reduced by:
- Covering cuts or lesions with waterproof dressings
- Careful cleansing of any injuries sustained during procedures
- Good personal hygiene
- Use of appropriate protective clothing
Staff should advise relatives of the precautions following viewing and/or physical contact with the deceased and when this should be avoided. Washing and/or dressing of the deceased should be avoided if the deceased is known or suspected to have an invasive streptococcal infection, viral haemorrhagic fevers or other Group 4 infectious agents.
Procedure
- Standard Infection Control Precautions should be used in caring for all deceased patients. This will include the use of plastic aprons and disposable gloves. Patients with a particular infection hazard should be identified to the mortuary staff or funeral directors to ensure that the appropriate precautions are taken in the ongoing care of the body.
- Exceptional care must be taken to avoid splashing body fluids if redressing wounds and removing urinary or intravenous catheters. Eye or face protection is recommended when splashing is likely.
- Viewing by relatives should take place before the body leaves the ward. If this is not possible, viewing of infected bodies may take place in the mortuary/viewing room (Refer to the Table). If relatives become distressed because they cannot view a body, the medical staff that cared for the deceased should be asked to discuss the matter with them. In the case where relatives or religious representatives wish to be involved in the performance of last offices, including hygienic preparation of the body or religious rites, on a patient who presents an infection hazard, advice may be obtained on an individual patient basis from the IPC team in the hospital or via the Public Health Agency duty room 0300 555 0119.
- Cadaver bags should be used where the containment of blood and body fluids is difficult or where there is a particular infection hazard (Refer to the Table for details of viewing restrictions)
- Post-mortem examination, if considered necessary for a patient with an infection hazard, must only be carried out in the Regional Forensic Mortuary in the Belfast Health and Social Care Trust (ADD CONTACT NUMBER).
Tuberculosis (Open Pulmonary Only)
- Patients with clinically suspected or diagnosed open pulmonary tuberculosis who have not completed two weeks of Chemotherapy are considered infectious.
- When the movement of the body is essential, a disposable cloth should be placed over the mouth and nose to prevent the release of aerosols of infectious materials.
- Staff must also wear appropriate respiratory protection when performing any procedures or moving the patient. This is especially important in the case of MDR-TB/XDR-TB.
- The clinical team looking after a patient have a duty to inform mortuary staff, funeral directors or embalmers about patients who present a particular infection hazard, particularly tuberculosis.
Category 4 Pathogens
Category 4 pathogens cause several rare infections. They are defined as a biological agent that causes severe human disease and is a serious hazard to employees; it is likely to spread to the community, and no effective prophylaxis or treatment is usually available (Refer to High-consequence Infectious Diseases). For example, Viral haemorrhagic fevers (Ebola, Marburg, Lassa fever, Crimean-Congo hemorrhagic fever), pulmonary anthrax etc. Refer to the Health and Safety Executive-approved list of biological agents for the categorisation of microorganisms.
Deceased individuals known or suspected to have a Hazard Group 4 infectious agent should be placed in a sealed double plastic body bag with absorbent material placed between each bag. The surface of the outer bag should be disinfected with 1000ppm av. cl before being placed in a robust sealed coffin. Post-mortem examination should not be performed on a deceased individual known or suspected to have Hazard Group 4 infectious agents.
Patients suffering from these and other dangerous diseases should be strictly isolated and transferred to the Regional Infectious Diseases Unit Belfast Health and Social Care Trust, Royal Hospitals site. Although there are no appropriate isolation facilities for these patients in most hospitals, a patient may be admitted and die before transfer. If the patient is suspected to be infected with a Category 4 pathogen, special precautions must be taken with the body. Advice must be sought urgently from the IPC team or the PHA duty room 0300 555 0119 if any of these diseases are suspected.
Table 1: Guidelines for handling cadavers with transmissible infections and microorganisms
Infectious diseases | Body bag | Viewing | Hygiene preparation | Embalming | |
Acute encephalitis | No | Yes | Yes | Yes | |
Anthrax | Adv | No | No | No | |
Acute poliomyelitis | No | Yes | Yes | Yes1 | |
Cholera | No | Yes | Yes | Yes1 | |
Chickenpox/shingles | No | Yes | Yes | Yes | |
Chikungunya | No | Yes | Yes | Yes | |
Covid-19 | Yes | Yes | No | No | |
Cryptosporidiosis | No | Yes | Yes | Yes | |
Carbapenem-resistant Enterobacterales (CRE) | No | Yes | Yes | Yes | |
Dermatophytosis | No | Yes | Yes | Yes | |
Diphtheria | Adv | Yes | Yes | Yes1 | |
Dysentery (bacillary) | Adv | Yes | Yes | Yes | |
ESBL-producing bacteria | No | Yes | Yes | Yes | |
Food poisoning | No | Yes | Yes | Yes | |
Hepatitis A | No | Yes | Yes | Yes | |
Hepatitis B and C | Yes | Yes | Yes | No | |
HIV | Yes only if bleeding | Yes | Yes | No | |
Invasive Group A streptococcal infection | Yes | Yes | No | No | |
Lyme disease | No | Yes | Yes | Yes | |
Legionellosis | No | Yes | Yes | Yes | |
Leptospirosis (Weil’s disease) | No | Yes | Yes | Yes | |
Measles | No | Yes | Yes | Yes | |
Meningitis (except meningococcal) | No | Yes | Yes | Yes | |
Meningococcal meningitis with or without septicemia | No | Yes | Yes | Yes1 | |
Malaria | No | Yes | Yes | Yes | |
MERS-CoV/SARS | Adv | Yes | Yes | No | |
Mumps | No | Yes | Yes | Yes | |
Mpox ( Monkeypox) | Adv | Yes | No | No | |
Ophthalmia neonatorum | No | Yes | Yes | Yes | |
Orf | No | Yes | Yes | Yes | |
Psittacosis | No | Yes | Yes | Yes | |
Plague | Yes | No | No | No | |
Q fever | No | Yes | Yes | Yes | |
Staph aureus, including MRSA (methicillin-resistant Staph. aureus) | No | Yes | Yes | Yes | |
Rubella | No | Yes | Yes | Yes | |
Relapsing fever | Adv | Yes | Yes | Yes | |
Rabies | Yes | No | No | No | |
Scarlet fever | Adv | Yes | Yes | Yes1 | |
Tuberculosis | Adv | Yes | Yes | Yes1 | |
Typhoid fever | Adv | Yes | Yes | Yes | |
Typhus | Adv | No | No | No | |
Tetanus | No | Yes | Yes | Yes | |
Transmissible spongiform encephalopathies (e.g., CJD and variant CJD) | Yes | Yes | Yes | No | |
Typhoid and paratyphoid fever | Adv | Yes | Yes | Yes | |
Viral haemorrhagic fever (Ebola, Marburg, Lassa fever, CCHF) | Yes | No | No | No | |
Whooping cough | No | Yes | Yes | Yes | |
Yellow fever | Yes | No | No | No | |
Zika | No | Yes | Yes | Yes | |
1 Requires particular care during embalming. Adv: advisable and may be required by local health regulations; Bagging: placing the body in a plastic body bag; Viewing: allowing the bereaved to see, touch, and spend time with the body before disposal; Embalming: injecting chemical preservatives into the body to slow the process of decay. Cosmetic work may be included; Hygienic preparation: washing, dressing, and tidying the body to present a suitable appearance for viewing.
REFERENCES
- HTM 03-01. Specialised ventilation for healthcare buildings. London: NHS England and NHS Improvement, 2021.
- Literature reviews: Transmission Based Precautions can be found in the definitions of Transmission Based Precautions.
- Health Building Note 04-01 (Supplement 1) Isolation facilities for infectious patients in acute settings. London: Department of Health, 2013.
- National infection prevention and control manual (NIPCM) for England.London: NHS England.
- Standard precautions for the prevention and control of infections: aide-memoire. Geneva: World Health Organization, June 2022.
- Transmission-based precautions for the prevention and control of infections: aide-memoire. Geneva: World Health Organization, June 2022.
- Damani N. Manual of Infection Control Procedures. 4th Oxford: Oxford University Press, 2019.
- Health and Safety Executive. Managing infection risks when handling the deceased. Guidance for the mortuary, post-mortem room, and funeral premises, and during exhumation. Norwich: The Stationery Office, 2018.
- Health and Safety Executive. The approved list of biological agents. Advisory Committee on Dangerous Pathogens. Health and Safety Executive, 2021.
- Hoffman PN and Healing TD. Guide To Infection Control In The Healthcare Setting.The Infection Hazards of Human Cadavers. International Society for Infectious Diseases. Updated: February 2022.
- HSG283 – managing infectious risk when handling the deceased.
- Control of substances hazardous to health (COSHH). Health & Safety Executive
- CDC and ICAN. Best Practices for Environmental Cleaning in Healthcare Facilities in Resource-Limited Settings. Atlanta, GA: US Department of Health and Human Services, CDC; Cape Town, South Africa: Infection Control Africa Network; 2019.
- National Standards of Healthcare Cleanliness 2021. London: NHS England and NHS Improvement, 2021.
